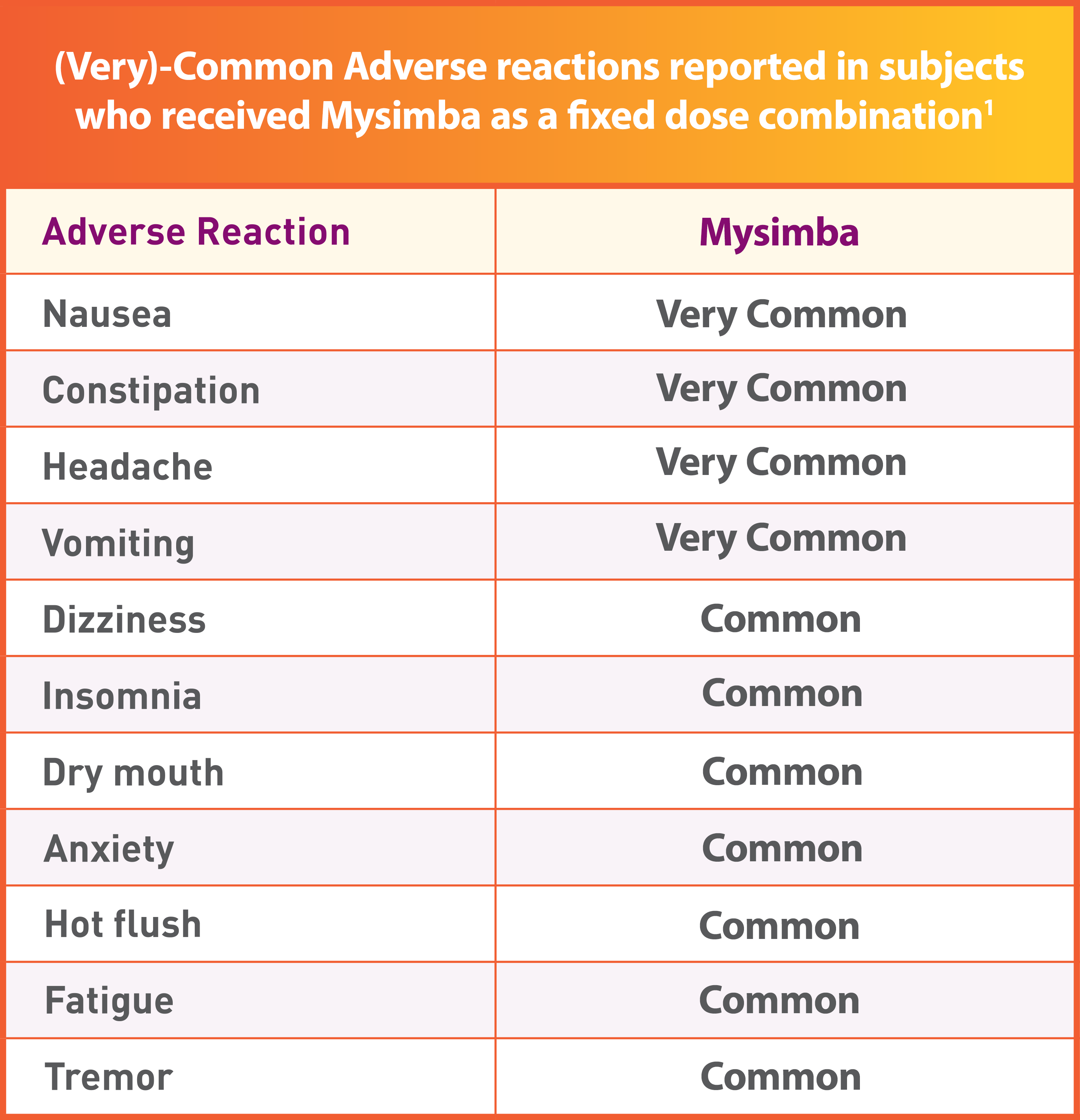
Tolerability demonstrated in thousands of patients1
- 24% of patients receiving Mysimba® and 12% of patients receiving placebo discontinued treatment because of an adverse reaction1
- The most frequent adverse reactions leading to discontinuation were nausea (Very Common), headache (Very Common), and vomiting (Very Common)1
- Common GI-related adverse reactions such as nausea and vomiting were generally transient in nature and resolved within 4 weeks for most patients.1-4
Please refer to the Mysimba® Summary of Product Characteristics (SmPC)
▼ Black Triangle
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Please refer to the Mysimba® Summary of Product Characteristics (SmPC)
▼ Black Triangle
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Please refer to the Mysimba® Summary of Product Characteristics (SmPC)
▼ Black Triangle
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
References:
- Mysimba® (naltrexone HCl and bupropion HCl) Summary of Product Characteristics (SmPC).
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605.
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19(1):110-120.
- Hong K, Herrmann K, Dybala C, Halseth AE, Lam H, Foreyt JP. Naltrexone/bupropion extended release-induced weight loss is independent of nausea in subjects without diabetes. Clin Obes. 2016;6(5):305-312.
