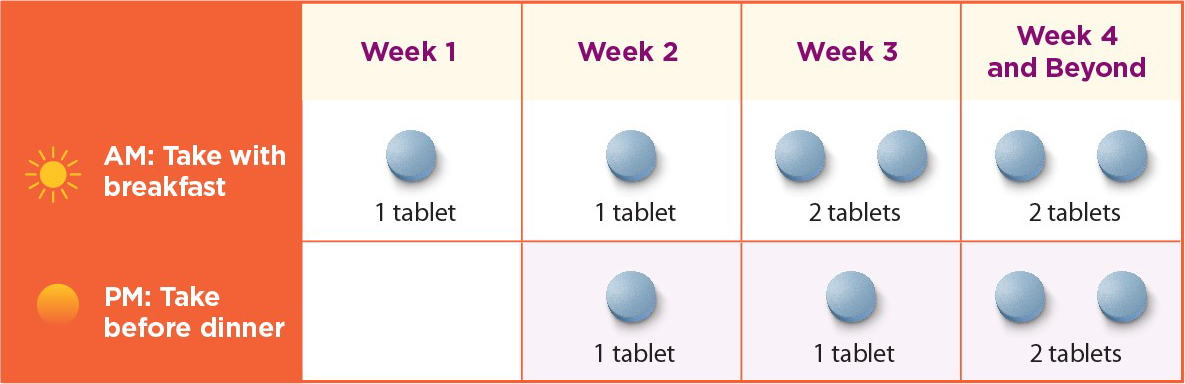
Mysimba® Allows for Full Titration Within 1 Month
Standard posology for Mysimba® (8 mg/90 mg)1‡
- Mysimba® is a convenient, oral option in combination with diet and exercise1
- Mysimba® is not a controlled drug or stimulant1,2
Not actual tablet size.
‡Dose adjustments are needed for patients with moderate, severe, or end-stage renal impairment, and hepatic impairment. Maximum daily doses are as follows: Moderate or severe renal impairment: 2 tablets per day (1 in the AM, 1 in the PM). Mild hepatic impairment: 2 tablets per day (1 in the AM, 1 in the PM). End-stage renal disease or severe hepatic impairment: contraindicated. Moderate hepatic impairment: not recommended for use in these patients.
Mysimba® and antidepressants1
- Coadministration of Mysimba® with certain antidepressants, including selective serotonin reuptake inhibitors (SSRIs) and many tricyclics, should be approached with caution
- The antidepressant should be initiated at the lower end of the dose
- Use extreme caution when coadministering Mysimba® with drugs that lower the seizure threshold
- If Mysimba® is prescribed to a patient already taking certain antidepressants, including SSRIs, dose adjustment has to be considered
- Mysimba® is contraindicated during or within 14 days of taking MAOIs
- Use of any other bupropion-containing products is contraindicated in patients taking Mysimba®
MAOI=monoamine oxidase inhibitor.
Please refer to the Mysimba® Summary of Product Characteristics (SmPC)
▼ Black Triangle
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Please refer to the Mysimba® Summary of Product Characteristics (SmPC)
▼ Black Triangle
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Please refer to the Mysimba® Summary of Product Characteristics (SmPC)
▼ Black Triangle
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
References:
- Mysimba® (naltrexone HCl and bupropion HCl) Summary of Product Characteristics (SmPC).
- QSYMIA [prescribing information]. Campbell, CA: VIVUS, Inc; 2017.
