COR-Behavioural Modification (BMOD) Study
Mysimba® Is Proven to Help Patients Lose Weight and Keep It Off
In COR-BMOD, patients participated in 28 group counseling sessions over 56 weeks and received individualized daily caloric goals and a prescribed exercise regimen1,2
- 80% of patients taking Mysimba® achieved ≥5% weight loss vs 60% taking placebo (completers)1
- 46% of patients taking Mysimba® achieved ≥5% weight loss vs 34% taking placebo (ITT-BOCF)1
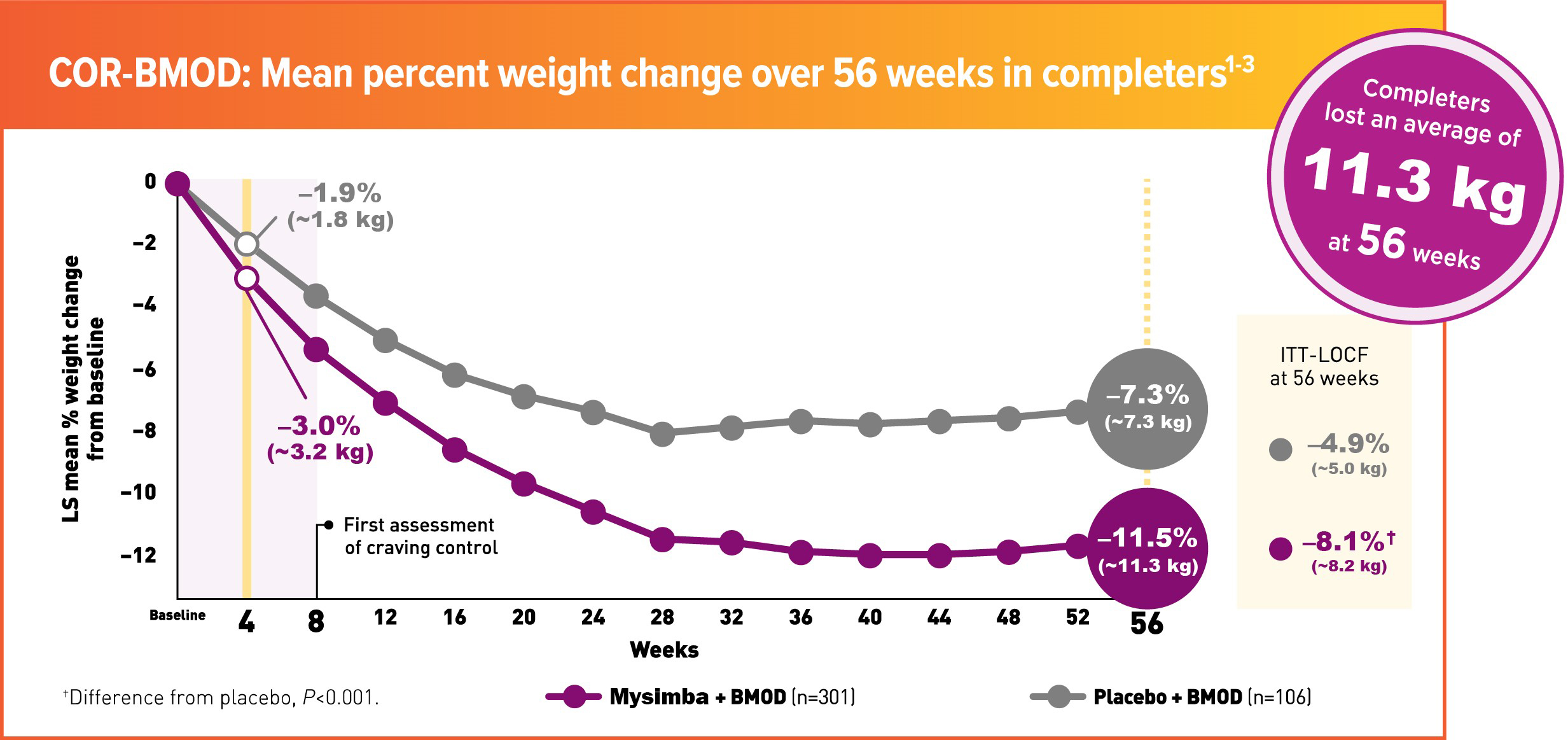
41.6% in the placebo group and 42.1% in the Mysimba® group discontinued study drug.
ITT=Intention-To-Treat; LOCF=last observation carried forward; LS=least squares; BOCF=baseline observation carried forward; COR=Contrave obesity research.
COR-BMOD was a 56-week, multicentre, double-blind, placebo-controlled study. Patients were randomised to Mysimba® 32 mg/360 mg daily or placebo. The co-primary endpoints were percent change from baseline body weight and the proportion of patients achieving ≥5% reduction in body weight at Week 56. Unless noted otherwise, data shown are from the ITT analysis, which included all randomised patients who had body-weight measurements at baseline and at least once post-baseline. LOCF was used for missing data.1,2
Patients received intensive behavioural modification by registered dietitians, behavioural psychologists, or exercise specialists, as well as a prescribed rigorous diet and exercise regimen. Patients met in groups of 10-20 people for 90 minutes. Group meetings were held weekly for the first 16 weeks, every other week for the next 12 weeks, and monthly thereafter (28 sessions in total).1,2
Mysimba® ITT: n=565, placebo ITT: n=196. Average baseline parameters were: Mysimba®: 100.2 kg, 109.2-cm waist circumference; placebo: 101.6 kg, 109.2-cm waist circumference. Patients who completed 56 weeks of treatment: Mysimba®: 57.9%; placebo: 58.4%.1
COR-Diabetes StudyPlease refer to the Mysimba® Summary of Product Characteristics (SmPC)
▼ Black Triangle
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Please refer to the Mysimba® Summary of Product Characteristics (SmPC)
▼ Black Triangle
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Please refer to the Mysimba® Summary of Product Characteristics (SmPC)
▼ Black Triangle
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
References:
- Mysimba® (naltrexone HCl and bupropion HCl) Summary of Product Characteristics (SmPC).
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011(1);19:110-120.
- Data on file.
